ED-4-4
Viscosity dependence of time evolution of aggregation of magnetic nanoparticles evaluated by HTS-SQUID AC magnetometer
18:00-18:15 28/11/2023
*Kei Yamashita, Rekka Moriya, Taiki Yamamoto, Shu Asayama, Rikuya Korenaga, Kanako Ogawa, Jin Wang, Toshihiko Kiwa
Graduate School of Interdisciplinary Science and Engineering in Health Systems, Okayama University, 3-1-1, Tsushima-Naka, Kitaku, Okayama, 700-8530, Japan.
Blood coagulation tests are in increasing demand as preoperative tests to evaluate hemostatic function. In blood coagulation tests, coagulant CaCl2 solutions are mixed with blood to accelerate coagulation and measure the coagulation speed. Magnetic nanoparticles (MNPs) have been developed for medical purposes such as hyperthermia and magnetic particle imaging. MNPs respond to the applied AC-magnetic field by rotating themselves, called Brownian relaxation. The time taken to respond to the applied AC-magnetic fields by the Brownian relaxation depends on the solvent viscosity and the particle volume of MNPs. Taking advantage of the magnetic response of MNPs depending on the viscosity, we have been developing a method to evaluate coagulation by detecting the changing signal of a mixture of MNPs. However, since MNPs are hydrophilic colloids, in blood coagulation tests, MNPs cause aggregation by shielding the surface charge by the calcium ions (Ca2+) ions in case of adding the aqueous CaCl2 solution. The magnetic signal of MNPs in blood coagulation tests is thought to decrease due to increased viscosity caused by coagulation in addition to the aggregation by the aqueous CaCl2 solution. Therefore, it is necessary to divide the influence of aggregation and coagulation to evaluate the magnetic signal of MNPs in blood coagulation tests. In this study, we evaluated the viscosity dependence on the time evolution of the magnetic signal of MNPs in aggregating using aqueous glycerol and CaCl2 solutions.
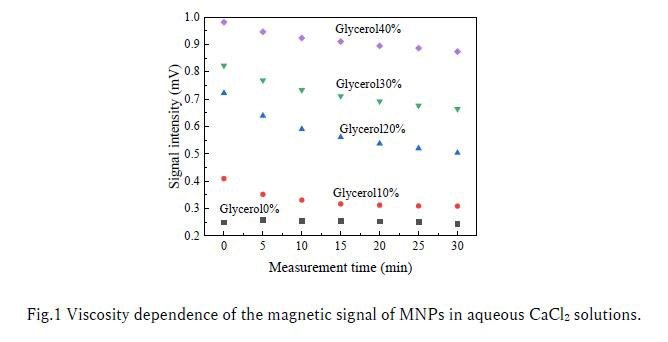
The HTS-SQUID AC magnetometer, as reported [1], was used to measure the magnetic signal of MNPs. This system utilized the third-harmonic magnetic signal to the applied magnetic field to separate the linear magnetic signal of water solvents and the non-linear magnetic signal from MNPs. As reported in [2], the system's instability was ±3 µV in the standard deviation. Fig.1 shows the time evolution of the magnetic signal of Resovist® in aqueous CaCl2 solutions. In addition, the magnetic signal of Resovist® in pure water was 1.04 mV. Resovist® was used as MNPs, an aqueous CaCl2solution was used as MNPs flocculant, and an aqueous glycerol solution was used as a thickener. The concentration of CaCl2 was 45 mmol/L, the iron concentration of Resovist® was 279 µg/mL, and the sample volume was 50 µL. The measurement was started 2 minutes after adding Resovist® to the mixture of aqueous CaCl2 and glycerol solution. As shown in the glycerol0% sample, the magnetic signal of Resovist® decreased due to aggregation caused by Ca2+ ions. However, comparing the magnetic signal of Resovist® in pure water with the magnetic signal of Resovist® in 0-40% glycerol after 30 minutes, the magnetic signal intensity increased to 23, 30, 48, 64, and 84%, respectively. Thus, Fig.1 shows the increasing magnetic signal of MNPs attributed to increasing viscosity. The tendency for increasing the magnetic signal as the glycerol concentration increases could be aggregation inhibition due to increasing viscosity. In blood coagulation tests, since coagulation does not occur in the plasma of hemophiliacs, the time evolution of the magnetic signal of MNPs can be affected only by aggregation. In contrast, in a healthy person's plasma, coagulation suppresses aggregation, and increasing viscosity due to coagulation is dominant in the time evolution of the magnetic signal of MNPs. Therefore, the result suggested that the hemostatic function can be assessed by evaluating whether coagulation hinders the aggregation of MNPs from the magnetic signal.
[1] T. Mizoguchi et al., "Highly sensitive third-harmonic detection method of magnetic nanoparticles using an AC susceptibility measurement system for liquid-phase assay," IEEE Transactions on Applied Superconductivity, vol. 26, no. 5, pp. 1-4, 2016.
[2] K. Yamashita et al., "Aggregation of Magnetic Nanoparticles in Biological Solvents Evaluated by HTS-SQUID Magnetic Immunoassay System," IEEE Transactions on Applied Superconductivity, vol. 33, no. 5, pp. 1-5, 2023, doi: 10.1109/tasc.2023.3239830.
This work was supported by JST SPRING, Grant Number JPMJSP2126.